I thought I would share my extreme ozone fade test results. They are only for comparative testing and give no absolute estimate of the life of prints. It was a quick and dirty experiment so keep that in mind and don't take the results too seriously.
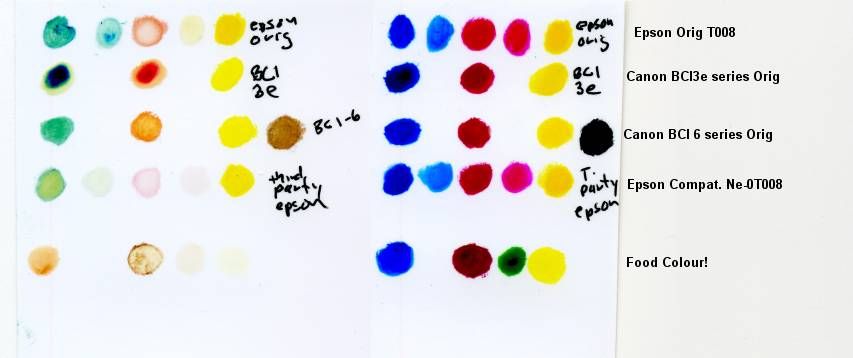
Left Ozone Exposed 30 min Right Room Air Exposed 24 hrs
Notes:
Conditions:
Exposure: 30 minutes extreme Ozone level Exposure
Environment: Small Closed Glass Jar with small ED Ozone generator producing a very high concentration, Room Temperature
Paper Epson Premium Glossy Photo Paper (microporous ceramic coating)
Ink dried for 24 hrs
-I had no T007 Black ink on hand to test
-The inks were not printed on but dabbed on with a cotton swab since several of the inks tested were from old machines that I no longer have a printer for.
Results:
The ozone level was very high but I do not know the actual level. It is the relative fading that is important.
The left half was placed in the ozone, and the right half placed in a room with no sunlight exposure.
The BCI3e cyan and magenta went on too thick so only look at the edge of those to rate the fading
The Epson Compatible came in a small green box. Manufacturer unknown but the code name is there on the photo legend.
Fade Level Best to Worst:
1-epson orig T008
2-Canon BCI -6
3 Canon BCI 3e and epson compatible T008 are at a tie in that the canon was better on magenta but the epson compatible was better on the cyan
4 -Food colour -added just for fun!
I also placed a Fuji crystal archive glossy print , and a magazine glossy photo in the jar -there was no visible fading of either of these.
Caveats: Ink density was hard to make consistent
This test was just to compare dyes with extreme ozone exposure and does not convey any UV susceptibility.
The Fuji crystal archive likely has a sealer coating as it does not feel like typical gelatin coatings when touched with a wet finger- not sticky. The magazine print I do not know much about but it is glossy and may have used pigments but I am not sure.
Many modern ink-jet papers use a microporous surface coating. These are often a nano particle coating such as Alumina. If you are not sure, take a small piece and burn it -a white or glossy brittle layer will remain because this ceramic doesn't decompose from heat. Problem with these is they let gases into the pores and these gases such as ozone, NOx, and SOx will oxidize and therefore fade most dyes. That is waht you are looking at in the test.
What about sprays to seal the pores?
What a difficult question. In theory they should help, but there can be problems with yellowing, interaction with dyes, and in the case of microporous ceraamics, the solvents can cause weakening of the binder that is used to bond the ceramic to the substrate which could eventually lead to cracking and peeling of this microporous layer.
Overall I am not a fan of microporous paper. Eventually I will try one of the swellable polymer types and see if it fairs better. Kodak seems to claim one of their's has a longer life but I do not believe 100 years. Ill try that one next .
Email me if you want me to run a test for you but I would need the test prints mailed.

Left Ozone Exposed 30 min Right Room Air Exposed 24 hrs
Notes:
Conditions:
Exposure: 30 minutes extreme Ozone level Exposure
Environment: Small Closed Glass Jar with small ED Ozone generator producing a very high concentration, Room Temperature
Paper Epson Premium Glossy Photo Paper (microporous ceramic coating)
Ink dried for 24 hrs
-I had no T007 Black ink on hand to test
-The inks were not printed on but dabbed on with a cotton swab since several of the inks tested were from old machines that I no longer have a printer for.
Results:
The ozone level was very high but I do not know the actual level. It is the relative fading that is important.
The left half was placed in the ozone, and the right half placed in a room with no sunlight exposure.
The BCI3e cyan and magenta went on too thick so only look at the edge of those to rate the fading
The Epson Compatible came in a small green box. Manufacturer unknown but the code name is there on the photo legend.
Fade Level Best to Worst:
1-epson orig T008
2-Canon BCI -6
3 Canon BCI 3e and epson compatible T008 are at a tie in that the canon was better on magenta but the epson compatible was better on the cyan
4 -Food colour -added just for fun!
I also placed a Fuji crystal archive glossy print , and a magazine glossy photo in the jar -there was no visible fading of either of these.
Caveats: Ink density was hard to make consistent
This test was just to compare dyes with extreme ozone exposure and does not convey any UV susceptibility.
The Fuji crystal archive likely has a sealer coating as it does not feel like typical gelatin coatings when touched with a wet finger- not sticky. The magazine print I do not know much about but it is glossy and may have used pigments but I am not sure.
Many modern ink-jet papers use a microporous surface coating. These are often a nano particle coating such as Alumina. If you are not sure, take a small piece and burn it -a white or glossy brittle layer will remain because this ceramic doesn't decompose from heat. Problem with these is they let gases into the pores and these gases such as ozone, NOx, and SOx will oxidize and therefore fade most dyes. That is waht you are looking at in the test.
What about sprays to seal the pores?
What a difficult question. In theory they should help, but there can be problems with yellowing, interaction with dyes, and in the case of microporous ceraamics, the solvents can cause weakening of the binder that is used to bond the ceramic to the substrate which could eventually lead to cracking and peeling of this microporous layer.
Overall I am not a fan of microporous paper. Eventually I will try one of the swellable polymer types and see if it fairs better. Kodak seems to claim one of their's has a longer life but I do not believe 100 years. Ill try that one next .
Email me if you want me to run a test for you but I would need the test prints mailed.